Introduction
Batteries come in many different Types of Batteries and sizes, and each type has its advantages and disadvantages. The most common types of batteries are lead-acid, nickel-cadmium, nickel-metal-hydride, and lithium-ion, Batteries are an essential part of our modern world. Without them, many of the devices we rely on would not be possible.
What is the battery?
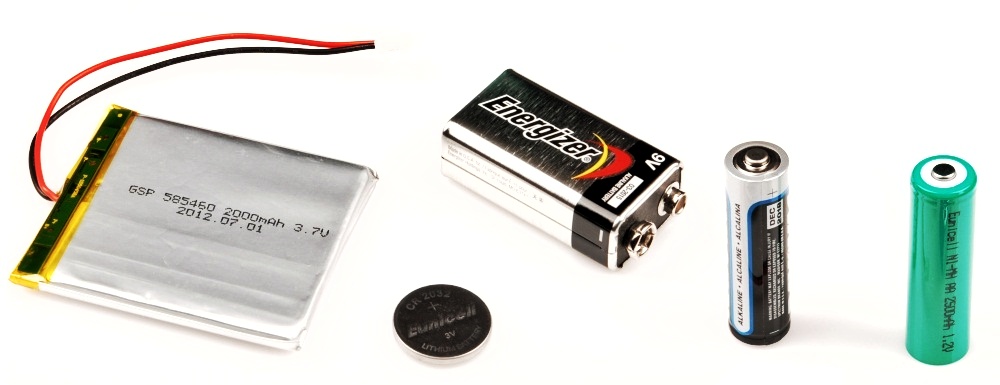
This is a battery A battery is a device that stores electrical energy and converts it into a form that can be used by an electronic device. The chemical reaction inside the battery creates a voltage, which is used to power electrical devices.
The first battery was invented in 1800 by Alessandro Volta. Volta’s battery was the first practical device for generating electricity. Today, batteries are an essential part of our lives, powering everything from cell phones to laptops to electric cars. Batteries are made up of three parts, the anode, the cathode, and the electrolyte. The electrolyte is the medium that allows for the flow of electrons between the anode and cathode.
The anode is the negative side of the battery and the cathode is the positive side. The chemical reaction that takes place in a battery is known as an electrochemical reaction. This reaction produces electricity that can be used to power devices
Battery cells are usually made up of three main components;
- The Anode (Negative Electrode)
- The Cathode (Positive Electrode)
- The electrolytes
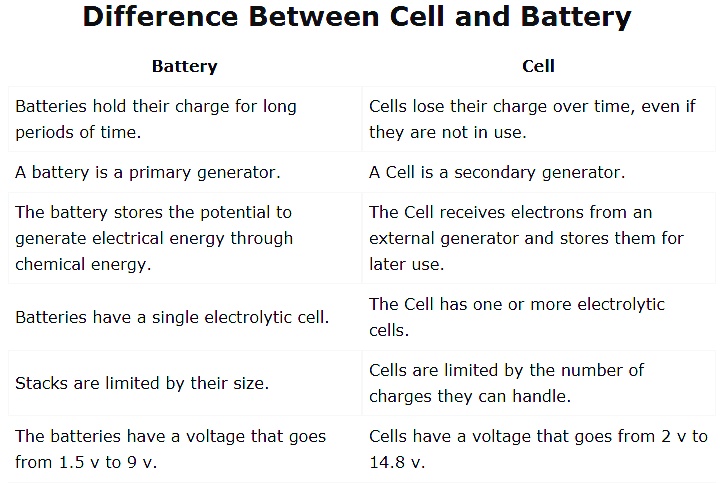
Difference between Cell and Battery
The cell is the basic unit of any electrical system. the cell provides the power for the system to operate. The battery is a device that stores and provides the cell with electrical energy.
1. Cell
The cell is the basic unit of any electrical system. It consists of one or more electrochemical cells that convert chemical energy into electrical energy. The cell provides the power for the system to operate.
2. Battery
The battery is a device that stores and provides the cell with electrical energy. It consists of one or more electrochemical cells that convert chemical energy into electrical energy. The battery provides the cell with a constant supply of electrical energy.
Types of Batteries
Batteries generally can be classified into different types and types, ranging from chemical design, size, and uses, but under all of these are two main battery types;
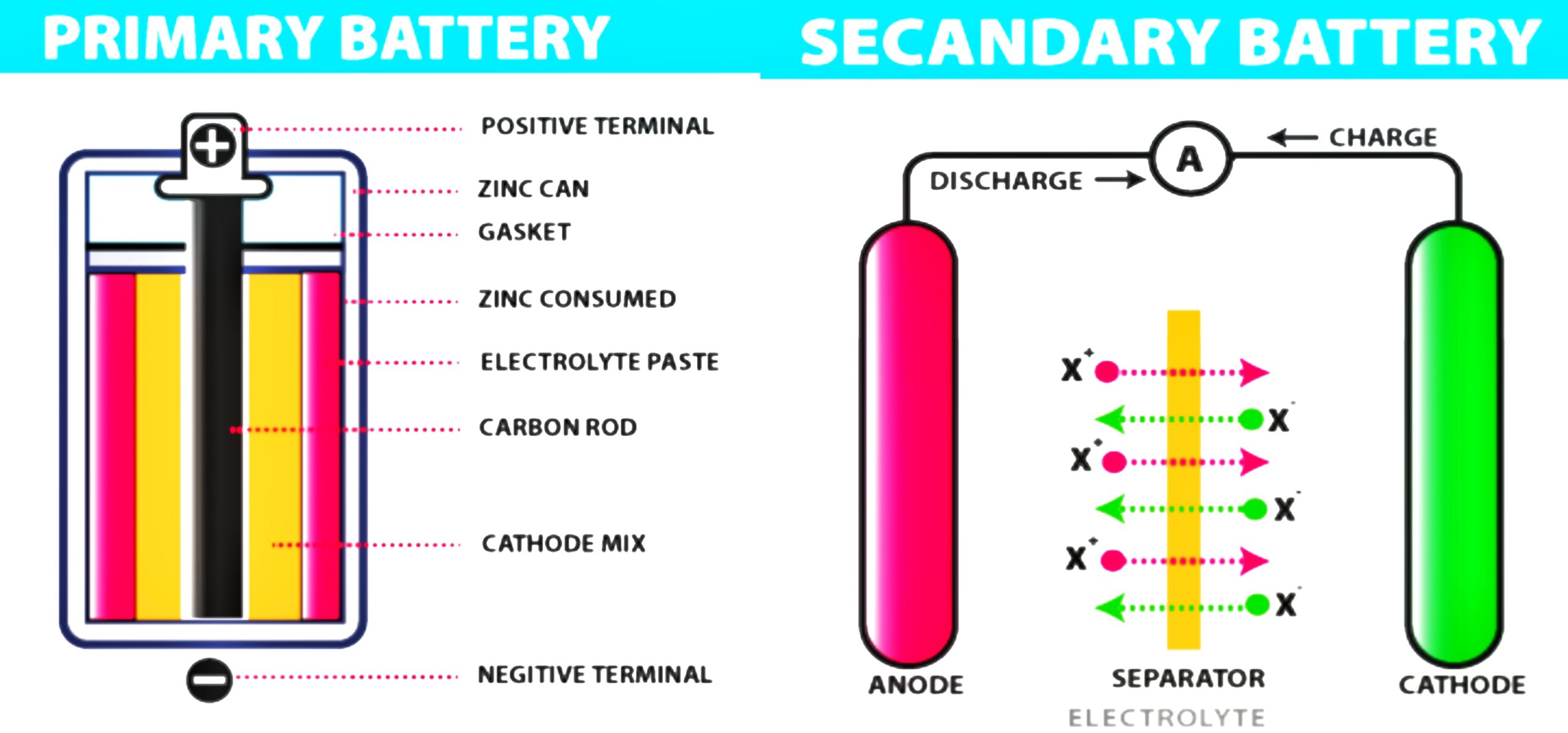
- Primary Batteries // Primary batteries are non-rechargeable batteries. This means it can’t be recharged once depleted.
- Secondary Batteries // The secondary batteries work otherwise. They’re ideal for recharging.
1. Primary Batteries
Primary batteries are batteries that cannot be recharged once drained. Primary batteries are made of electrochemical cells whose electrochemical reactions cannot be inverted. Primary batteries are discovered in many common Electronics products such as remote-control toys, analog watches, flashlights, portable entertainment devices, etc.
The following table shows various types of primary batteries along with their features and applications.
| Battery Type | Characteristics | Applications |
| Zinc – Carbon | Common, low cost, variety of sizes | Radios, toys, instruments |
| Magnesium (Mg/MnO2) | High capacity, long shelf life | Military and aircraft Radios |
| Mercury (Zn/HgO) | Very high capacity, long shelf life | Medical (hearing aids, pacemakers), photography |
| Alkaline (Zn/Alkaline/MnO2) | Very popular, moderate cost, high performance | Most popular primary batteries |
| Silver/Zinc (Zn/Ag2O) | Highest capacity, costly, flat discharge | Hearing aids, photography, pagers |
| Lithium/Soluble Cathode | High energy density, good performance, wide temp range | Wide range of applications with capacity between 1 – 10,000 Ah |
| Lithium/Solid Cathode | High energy density, low temp performance, long shelf life | Replacement for button and cylindrical cells |
| Lithium/Solid Electrolyte | Low power, extremely long shelf life | Memory circuits, medical electronics |
Credit Electronics Hub.
2. Secondary Batteries
Secondary batteries are rechargeable batteries that have multiple applications in today’s world. They are generally used in electronic devices such as laptops, smartphones, and digital cameras. Secondary batteries have many benefits over primary batteries, which are not rechargeable. They can be recharged multiple times, which saves money and resources, and they last longer overall.
Different types of Secondary Batteries
The following are the different types of rechargeable batteries that are generally used.
- Lead-Acid
- Nickel Cadmium(Ni-Cd)
- Nickel-Metal Hydride(Ni-MH)
- Lithium-ion(Li-ion)
1. Lead–Acid Batteries

Lead-acid batteries are the oldest type of battery, and they are still used in many applications today. Lead-acid batteries have a high energy density and are very durable, but they are also very heavy and can be dangerous if not used properly.
Nickel – Cadmium Batteries
Nickel-cadmium batteries were once the most popular type of battery, but they have been replaced by newer technologies in most applications. Nickel-cadmium batteries have a high energy density and are very durable, but they are also very toxic and can be dangerous if not used properly.
Some of the properties of Nickel-Cadmium batteries are documented below.
- Specific Energy: 40-60W-h/kg
- Energy Density: 50-150 W-h/L
- Specific Power: 150W/kg
- Charge/discharge efficiency: 70-90%
- Self-discharge rate: 10%/month
- Cycle durability/life: 2000cycles
Nickel–Metal Hydride Batteries
Nickel-metal-hydride batteries are similar to nickel-cadmium batteries, but they are not as toxic. Nickel-metal-hydride batteries have a high energy density, but they can be expensive. They are used in some cordless power tools. Nickel-cadmium batteries are durable and have a long life.
Some of the properties of Nickel-metal hydride batteries
- Specific Energy: 60-120h/kg
- Energy Density: 140-300 Wh/L
- Specific Power: 250-1000 W/kg
- Charge/discharge efficiency: 66% – 92%
- Self-discharge rate: 1.3-2.9%/month at 20oC
- Cycle Durability/life: 180 -2000
Lithium-Ion batteries
Lithium-ion batteries are the newest type of battery, and they are quickly becoming the most popular type of battery. Lithium-ion batteries have a very high energy density, are very lightweight, and can be recharged very quickly. They are used in some electric cars. Lithium-ion batteries are lightweight, have a long life, and are environmentally, However, they can be expensive and can be dangerous if not used properly.
Some of the features of lithium-ion batteries are detailed below;
- Specific Energy: 100: 265W-h/kg
- Energy Density: 250: 693 W-h/L
- Specific Power: 250: 340 W/kg
- Charge/discharge percentage: 80-90%
- Cycle Durability: 400: 1200 cycles
- Nominal cell voltage: NMC 3.6/3.85V
Battery Applications
Batteries are a very important part of our everyday lives. They provide power for many of the devices we use a daily wide variety of devices, from cell phones to laptops to hybrid cars. The modern world would be very different without batteries.
- Mobile Devices. Batteries power a wide variety of modern items. …
- Cameras. …
- Small Household Items. …
- Home Appliances. …
- Portable Computers. …
- Toys and Games. …
- Cordless Tools. …
- Clocks and Watches.
Which type of battery is best for different purposes
There are many different types of batteries, each with its advantages and disadvantages. The best battery for a particular purpose depends on many factors, including the required voltage, the discharge rate, the operating temperature, and the size and weight of the device.
Nickel-cadmium batteries were once the most popular type of rechargeable battery, but have now been replaced by newer technologies. They are still used in some applications, such as power tools, because of their high power density and long lifespan.
However, they are expensive and have a negative environmental impact due to the cadmium content. Nickel-metal-hydride batteries are similar to nickel-cadmium batteries, but do not contain cadmium and are therefore more environmentally friendly.
They are often used in portable electronic devices, such as laptops and cell phones. They have a high power density and a long lifespan but are more expensive than lead-acid batteries.







1 Comment
Pingback: What is Sensor ? Different Types of Sensor - Sensors Explained.